- ICAR 2023 organizers have funding for students to attend. Applications due JANUARY 31, 2023.
- See their site for details on how to apply.
- Their Registration Fee Deadlines are:
- EARLY FEE: before Jan 31, 2023: approximately $300 - $550 USD (depends on exchange rate)
- MIDDLE FEE: Feb. 1- April 1, 2023: approximately $310 - $585 USD (depends on exchange rate)
- LATE FEE: April 2- June 9, 2023: approximately $335 - $625 USD (depends on exchange rate)
NAASC FUNDING FOR US SCIENTISTS ONLY- APPLICATIONS DUE: FEBRUARY 13, 2023
We have funding for about 20 US plant biologists to participate: Up to 10 awards in Category 1 (travel awards) and Up to 10 awards in Category 2 (virtual participation).
Note that Category 1 awards for in-person attendance will be insufficient to cover full participation costs. All additional costs are the responsibility of the attendee.
- Eligible:
- US plant biologists studying or employed in the USA (US citizenship not required).
- Applications from women and members of other underrepresented groups are strongly encouraged and diversity will be one key aspect of the selection process.
- And at least one of these:
- Early Career Scholar: students, postdocs in postdoc position, or pre-tenure faculty and/OR
- Member of underrepresented groups in US STEM (Native/African/Latinx American of Central/South American descent only) and/OR
- Faculty at HBCU/1890s/Black serving institutions or Tribal Colleges and Universities (TCU) that mentor members of underrepresented groups (Native/African/Latinx American of Central/South American descent only)
- Timeline
- February 13, 2023: Submit all materials (including letter of support for Category 1 students & postdocs)
- February 22, 2023: Awards announced by email to address provided
- February 28, 2023: Awardees must register and submit their abstract directly to ICAR 2023
- Awardee Requirements
- Awardees must present their work at ICAR (talk or poster)
- By 28 February, 2023: Awardees must register and submit their abstract directly to ICAR 2023 (submitting your abstract for the award application does not fulfill this requirement)
- By February 22- 6 days before the deadline- we will email all applicants as to whether they have been selected for an award.
- It is your responsibility to register and submit your abstract to ICAR 2023.
- You may want to wait for your notification of whether you have been selected for an award before registering and paying as they will not refund your fee if you change your mind due to not being selected for an award, for for any other reason.
- Failure to present your work at ICAR forfeits your award
- Awardees must submit a short meeting evaluation and survey after ICAR
- Awards given as reimbursements
- Awardees will sign an award offer noting these requirements sent by NAASC
-
Application Process for Eligible Applicants
-
Determine your category of award: Category 1: Travel Award: up to $1,000 USD reimbursement for valid conference expenses (receipt(s) required)
Category 2: VOPA (Virtual Online Participation Award): reimbursement of virtual registration fee (receipt required)
-
Assemble a full application package:
-
Completed Google form (includes: demographic questions, abstract title & description, and statement on how attending ICAR 2023 will impact you)
-
2-page CV or resume (pasted into Google form OR emailed*)
-
Category 1 students and postdocs only: Letter of support (LOS) emailed directly by letter writer to Joanna Friesner (see below*) Please ask your writer to include your name in the email subject and pdf filename. The LOS should indicate that the writer understands that travel awards will not fully cover all meeting costs and that awardees must cover all remaining costs.
-
*Email LOS (and CV/resume if not pasted into google form) to Dr. Joanna Friesner arabidopsisconference[at]gmail.com
Download PDF of information to apply for NAASC travel funding
NAASC Annual Election
The results of the 2022 election are below. We thank all community members that submitted nominations, and those that agreed to be on the ballot. We welcome the new members to serve on the community via NAASC, and we encourage others in the community to consider volunteering in the future.
We also thank Jennifer Nemhauser, outgoing NAASC president who concludes her 5 year term of service to the community.
5 year faculty member terms (2022-2027)
- Mentewab Ayelew (Spelman College, USA)
- Liang Song (University of British Columbia, Canada)
1 year Early Career Scholar member terms (2022-2023)
-
Arif Ashraf, (University of Massachussetts Amherst)
-
Luis de Luna (UC Riverside)
-
Margot Smit (Stanford University)
- Priorities for Incoming Faculty (5-year NAASC terms):
- engaging multidisciplinary approaches & increasing participation, diversity, and inclusion in all NAASC activities.
- participating in 1+ NAASC working groups including advocating for fundamental plant biology, ensuring NAASC sustainability, membership development & support, acting as allies to minoritized community members, and others that may be proposed by NAASC.
- developing &/or implementing NAASC's next community-serving funding proposal
- participating with the Early Career Scholars Subcommittee (ECSS) and/or Inclusivity Scholars Program (ISP, formerly named URM program); or propose & lead a new community-supportive subcommittee
- Priorities for Incoming ECS (grad students & postdocs, 1-year ECSS terms): ECSS priorities will fluctuate based on priorities, funding, & community input. Examples of priorities that ECSS members may engage with include developing ECS-focused activities for: ICAR 2024- San Diego (the next NAASC-run conference), communication with ECS (including e.g., by social media, zoom seminars, discussions, workshops), collaborating with the NAASC ISP (Inclusivity Scholars Program for minoritized plant biologists), networking, training, career development, personal & professional support, surveying the ECS community for priorities, and/or other important issues raised by the committee or the community.
The North American Arabidopsis Steering Committee (NAASC)
- Federica Brandizzi, Michigan State University (2018 - 2023) NAASC President
- Anna Stepanova, NC State University (2018 - 2023) NAASC Secretary
- Siobhan Braybrook, UCLA (2019 - 2024)
- Keith Slotkin, Donald Danforth Plant Science Center & Univ. of Missouri-Columbia (2019 - 2024) NAASC Vice President
- Cris Argueso, Colorado State University (2020 - 2025) NAASC Treasurer & Chair, Inclusivity Scholars Program (ISP)
- Adrienne Roeder, Cornell University (2020 - 2025) NAASC Representative to MASC
- Dior Kelley, Iowa State University (2021 - 2026) Co-chair, Early Career Scholars Subcommittee (ECSS)
- Glora Muday, Wake Forest University (2021 - 2026)
- Mentewab Ayalew, Spelman College (2022 - 2027)
- Liang Song, University of British Columbia (2022 - 2027)
- Joanna Friesner, unelected- NAASC Executive Director
**Immediate Past NAASC Members**
- Jennifer Nemhauser, University of Washington, Seattle (2017 - 2022)
- Roger Innes, Indiana University (2016-2021)
- Peter McCourt, University of Toronto (2016-2021)
- Sean Cutler, UC Riverside (2017-2021)
- Jose Dinneny, Stanford (2015 - 2020)
- Elizabeth Haswell, Washington University- St. Louis (2015 - 2020)
Click here to see ALL past serving NAASC members
NAASC Executive Director: Joanna Friesner (Executive Director, NAASC) has served as the NAASC staffperson since 2006, including four years as MASC Coordinator. She can be reached at [This email address is being protected from spambots. You need JavaScript enabled to view it.]. NAASC is primarily composed of U.S. researchers, and typically at least one Canadian researcher, all of whom are elected to five year terms of service by the North American Arabidopsis community. Current and Past NAASC Members are listed at the bottom of the page.
Click here to see full NAASC Structure and Subcommittee Descriptions
-
- NAASC- 10 elected members of the North American Arabidopsis community; serve 5 year terms; 2 new members elected annually
- DEILC- Diversity, Equity and Inclusion Leadership Committee, includes NAASC members, leads of other subcommittees, and Executive Director. Objective: to ensure NAASC uses a lens of social and racial equity and inclusion in its activities.
- Inclusivity Scholars Subcommittee (ISS) (formerly NAASC Minority Affairs Committee, MAC). Objective: to increase the presence of historically under-represented ethnic and racial groups in the fields of Arabidopsis research, education, and outreach, as well as to collaboratively address issues they face as plant scientists and in society.
- (new, fall 2021) Early Career Scholars Subcommittee (ECSS), Objective: to ensure that Early Career Scholars (ECS) have representation in NAASC activities and to provide a forum where we can collaboratively address issues specific to ECS.
- future subcommittees: NAASC is open to developing new subcommittees to represent communities or projects. Contact us to discuss your proposal: This email address is being protected from spambots. You need JavaScript enabled to view it.
August 29, 2017 Update to NAASC Term of Service: To provide better continuity and shared knowledge for NAASC-organized ICARs, current NAASC agreed, by consensus, to increase the term from four to five years. Current members will opt-in to a 5th year, while all NAASC elections beginning with fall, 2017, will designate 5 year terms for new NAASC members.
Category 1: IN-PERSON* Early Career Researcher (ECR) partial participation awards to US plant biologists that are students, postdocs in postdoc title, or pre-tenure junior faculty (and that are NOT a member of the groups under-represented in US science listed in 2) We will award up to $1,000 each, as reimbursements, for eligible ICAR costs; receipts must be submitted e.g. registration, lodging, travel, following NSF rules.
Awardees Selected by NAASC
- Gozde Demirer UC Davis Postdoc.
- Stephen Deslauriers University of Minnesota, Morris Junior Faculty (pre-tenure)
- Mona Gouran UC DAVIS PhD/Doctoral Student
- Grace Johnston Colorado State University Master's Student
- Eun-Deok Kim University of Texas at Austin Postdoc
- Gabrielle Rupp University of Missouri-Columbia PhD/Doctoral Student
- Martha Schwall Louisiana State University PhD/Doctoral Student
- Mingli Xu University of South Carolina Junior Faculty (pre-tenure)
- Eric Yang University of Washington PhD/Doctoral Student
Category 2: IN-PERSON* Inclusivity Scholars Program (ISP) full funding for up to 10 US plant biologists that are Native/African/Latinx American of Central/South American descent only; OR plant biology faculty at HBCU, 1890, Black-serving institution in the US and that mentor, teach, support members of under-represented groups. Full funding includes: coordinated registration, economy and travel, following NSF rules. Full details will be sent to selected awardees who will join the NAASC ISP Cohort for coordinated activities and travel. The vast majority of ISP participation costs will be paid up-front by NAASC.
Awardees Selected by NAASC
- David Bullock North Carolina State University PhD/Doctoral Student
- Norma Castro Guerrero University of Missouri Postdoc
- Efren Gonzalez Carnegie Institution for Science PhD/Doctoral Student
- Michael Guzman UC Riverside PhD/Doctoral Student
- Carlos Juarez Colorado State University PhD/Doctoral Student
- Stefanie King Washington University in St. Louis PhD/Doctoral Student
- Imani Madison North Carolina State University PhD/Doctoral Student
- Thelma Madzima University of Washington Bothell Faculty
- Marcel Malena New York University PhD/Doctoral Student
- Edith Pierre-Jerome Duke University Postdoc
- Carin Ragland Stanford University PhD/Doctoral Student
Category 3: VIRTUAL/ONLINE Participation Awards (VOPA) covering virtual registration fee *only* for US plant biologists that qualify in either/both of categories 1 or 2, and/or have financial need, and wish to participate virtually. Reimbursement requires submission of registration receipt to support actual costs paid. Registration fees are ~$120- $240 USD (depending on currency exchange at time of purchase) for early career and regular applicants, respectively. VOPA applicants do not need to submit a letter of support.
Awardees Selected by NAASC
- Ling Li Mississippi State University
- Pablo Jenik Franklin & Marshall College
-
NAASC Statement on Harassment and Discrimination (October 11, 2021)
- We stand with our colleagues in the Maize Genetics Cooperation in supporting targets of harassment and discrimination, and renew our pledge to uphold our code of conduct at ICAR meetings to prevent anyone from experiencing these harms.
- While current accusations against a member of our community are being investigated and the facts may not yet be fully known (see information here), we are aware that there may be many of our peers and mentees who have previously and/or continue to experience harassment and discrimination in their workplaces. Recent news stories are likely to renew their pain.
NAASC is committed to creating safe conferences, and to working within our community to eliminate behaviors which are harmful to the targets, undermine the integrity of the scientific enterprise, and rob us of precious potential contributions by the survivors of abuse.
Sincerely,
NAASC

ICAR 2021-Virtual had ~ 1000 participants from 40 countries
The first virtual ICAR was a success with 300+ speakers across 51 scientific session.
-
Summary of 6 novel approaches by NAASC, the ICAR 2021 organizers
Click here for pdf with details on approaches (scoring on scale of 1-4: 4=excellent, 3=good, 2=average, 1=poor)
- inclusion of 36 community-proposed and led mini-symposia
- selected invited plenary & keynote speakers, including through community nomination, that hadn't been featured in 5+ years
- developed new plenary session topics around bigger mechanistic themes; a balance of fundamental discoveries with work that showcases applied research & a focus on the broad set of tools of high utility available for Arabidopsis that enable translation to important crop species
- diversifying speakers in sessions & engaging non-traditional speakers including the prioritization of balanced demographics in speakers (gender & career stage
- dramatically increasing the number of opportunities (N=300+) for participants to present their work
- discussion sessions at end of concurrent symposia
Click here for ICAR 2021 Program and list of abstracts
- Attendees from nearly 250 institutions/companies
- 300+ speakers
- 50% increase (over average) of number of countries participants came from
- First time ICAR attendees: 61%
- Funders & Sponsors enabled nearly 1/3 of attendees to participate
- 2 Keynote sessions
- 7 Plenary sessions
- 36 community-led mini-symposia
- 6 community-led workshops
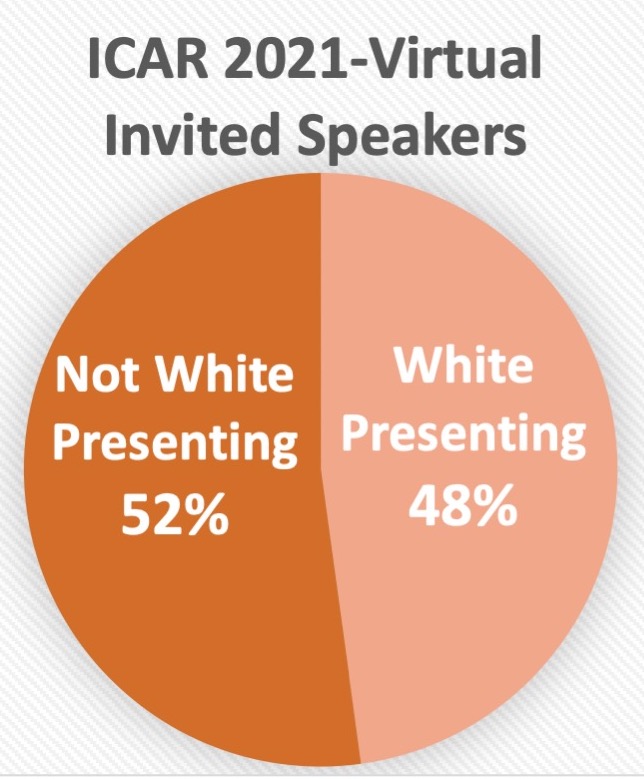
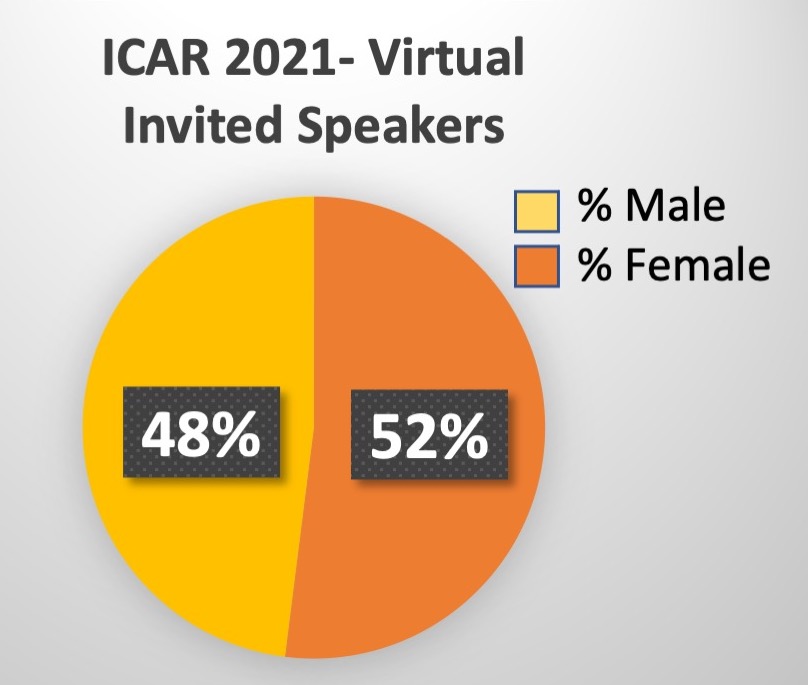
- Mini-symposium Speakers: 52% women, 44% men, 3% preferred not to say, 1% non-binary
- Mini-symposium Speakers: 40% postdocs and speakers; 27% junior faculty (pre-tenure); 26% senior faculty (post-tenure); 8% other (rounded)
- 24 invited platform speakers: 52% women, 48% men
- Mini-symposia, Keynote, and Plenary session talks were recorded and posted online 1 week prior to ICAR; remain accessible for 6 weeks post-ICAR
- Each session had a live Q&A and discussion component
ICAR 2021 Code of Conduct: All ICAR 2021 participants are expected to abide by the code of conduct: click here for pdf (full document)
include increasing participation by, and representation of, women, members of under-represented racial and ethnic groups in STEM, early-career researchers, and additional identities that span the full diversity of the human experience and our plant biology communities.

-
Specific areas we monitor for our activities have, so far, included: % female and/or early career stage invited speakers to ICARs.
-
Newer efforts will collect data on racial & ethnic identities and gender pronouns to enable us to gauge participation and effects of specific efforts on increasing representation and comfort level/"belongingness" of additional members of our community that have often been less/in-visible, and/or minoritized or excluded.



DiversifyPlantSci
-
- NAASC is committed to promoting a global plant sciences community that reflects the true diversity of all its members.
- To further this mission, members of the NAASC Diversity and Inclusion Task Force have created the DiversifyPlantSci online resource, a list of plant scientists from under-represented groups (see below) to reference as you seek speakers, reviewers, and participants for career or mentorship opportunities.
- This list (and database) is intended to highlight the diversity within the global plant science community.
- We hope to increase diversity and inclusion by making it easy to expand invitations past one’s personal networks.
How to Join and Use DiversifyPlantSci:
- Nominate yourself if you are a plant scientist who: identifies as a woman; identifies as LGBTQIA+; has a disability; and/or is a member of an under-represented ethnic or racial group (As defined by the US National Science Foundation: Women, persons with disabilities, and three racial and ethnic groups—blacks, Hispanics, and American Indians or Alaska Natives—are underrepresented in US STEM; we also include Filipino. https://www.nsf.gov/statistics/2017/nsf17310/digest/introduction/)
- You may also self-nominate if you otherwise belong to an underrepresented or minoritized group in academia (by your definition).
- You may include in the database and entry form any other unlisted identities that you wish to, that represent the unique you.
- Please do not nominate others, we allow self-registration only.
- However we strongly encourage you to forward the form to others that you know and invite them to consider signing up for inclusion.
- You may self-nominate by filling in the DiversifyPlantSci Self-Nomination Form
- We've used some language from the DiversityEEB list, and here's their reference: https://diversifyeeb.com/entries/
- Please do NOT use this database to "spam" many (or all) members as part of a broad or unfocused approach to reaching under-represented plant scientists. Use the list judiciously and keep in mind that a personal email is much more effective than a mass mailing. If you do send an email to more than one person, please use the 'bcc' option to reduce the likelihood of an annoying "reply-all" chain.
- For broad announcements, please instead make use of the NAASC [@DiversifyPlants] (https://twitter.com/diversifyplants) Twitter account to *broadly* announce, for example, career positions, award opportunities, or seminar announcements that aren't aimed at individuals you have *individually* identified as highly relevant to your activity. The intent of this database is to help enable "real" new connections and expand networks in meaningful ways. This will be accomplished being intentional and thoughtful about reaching out to folks on the list.
-
NAASC Member Blog, By Jennifer Nemhauser and Liz Haswell
-
-
ART-21 Publications
- The Next Generation of Training for Arabidopsis Researchers: Bioinformatics and Quantitative Biology. Plant Physiology, Volume 175, Issue 4, December 2017, https://doi.org/10.1104/pp.17.01490
- Directions for research and training in plant omics: Big Questions and Big Data. Plant Direct, Volume 3, Issue 4, April 2019, https://doi.org/10.1002/pld3.133
-
Broadening the impact of plant science through innovative, integrative, and inclusive outreach. Plant Direct, Volume 5, Issue 4, April 2021, https://doi.org/10.1002/pld3.316
ART-21 Presentations
-
"Seeds of Change: Using Plants to Broaden the Impact of Science in Society" NAASC-organized Symposium held November 7 2018 at UC Davis. The public symposium and the accompanying two day workshop were supported the US National Science Foundation* ART-21 award. These presentations can be found on the Plantae Website.
-
"Improving Outreach in Plant Science" workshop a ICAR 2021-Virtual, held June 21 2021. Organized by Jose Dinneny, former NAASC member and lead author on the related Plant Direct Outreach guide (listed above, April 2021)
You can view a brief overview of this activity as presented at PAG 2016 at: http://bit.ly/1QfMh4V
June 2015: NAASC member Siobhan Brady (PI, UC Davis) and NAASC Executive Director Joanna Friesner (co-PI, UC Davis), with contributions from several project Steering Committee members, were awarded 5 years of funding from the US National Science Foundation to support community engagement and activities focusing on research and training needs for Plant Biology in the 21st Century. www.nsf.gov/awardsearch/showAward?AWD_ID=1518280
Project's Three Main Objectives:
- Identify emerging technologies where using Arabidopsis as a model organism will provide fundamental discoveries and enable translational research in crop species
- Enhance interdisciplinary training of scientists for academia and extra-academic careers
- Increase diversity of Arabidopsis research scientists using targeted mechanisms
Major Project Activities
- Annual Focus Groups in each of 4 years (primarily involving North American plant biologists and several international community members) on three main topics:
- Computational training of biologists for academia and industry in the 21st Century
- Genomic experimental biology techniques for academia and industry in the 21st Century
- Interdisciplinary Training and Cross-training for 21st Century Careers
- *Wrap Up, Evaluation and Assessment and Write-Shop*
- Enabling Participation of US Scientists to attend ICAR (2015-2021):
- US Early Career Researcher (ECR) travel awards
- US Under-Represented Minority (URM) travel awards for historically under-represented groups in US science
- Community activities at each ICAR (2015-2021):
- Community workshops on each year's theme (matching the Focus Group)
- Interactive programs for community workshops involving early career researchers
- ICAR 2017 included an expanded list of activities involving research and training needs, aimed primarily at early-career researchers. These activities were made possible by the ART-21 RCN award from the NSF to NAASC, and through sponsorship and partnership with the Donald Danforth Plant Science Center (DDPSC) in St. Louis.
- Expanded ICAR 2017 Activities That Took Place in St. Louis, Missouri
ICAR 2017 Pre-Meeting Hands On Workshops for early career scientists:
The workshop content focused on emerging bioinformatic and computational skills and emerging genomics technologies. Activities included an full day workshops: Data Carpentry; phenotyping hackathon, and ATAC-seq lab:
- Data Carpentry: This event was an example-driven workshop on basic concepts, skills and tools for working more effectively with data. Short tutorials alternated with hands-on practical exercises, and participants were encouraged both to help one another, and to try applying what they have learned to their own research problems during and between sessions.
- ATAC-seq hands-on workshop on mapping chromatin accessibility and TF footprints: Instructor: Professor Roger Deal (Emory University)- This workshop introduced attendees to the ATAC-seq process including isolation of nuclei, preparation of ATAC-seq libraries, and analysis of the resulting data to identify open chromatin regions and TF footprints. This lab workshop included both wet-lab and computational components.
- Hackathon for high-throughput phenotyping: Designer: Cody Markelz (University of California, Davis). On-site Instructors: Malia Gehan and Noah Fahlgren (Donald Danforth Center). Many plant biologists are not formally trained to perform tasks needed to piece together their own image processing phenotyping pipeline. The hackathon focused on expanding on the introduction to genomics data and data management and analysis for genomics research provided in the Data Carpentry workshop.
- New Sessions: Quantitative and Computational Biology; Emerging Genomics Technologies.
- Special Community Workshops: URM career-development workshop abd ECR career-development workshop
Publications/ Information Sharing
- White Papers stemming from Focus Groups
- Webpage information dissemination
- Community Surveys
*This material is based upon work supported by the National Science Foundation under Grant No. #1518280. Any opinions, findings, and conclusions or recommendations expressed in this event are those of the presenters and do not necessarily reflect the views of the National Science Foundation.
Collaboration with ASPB on "Changing Climates and Cultures" (CCC)
The mission of Changing Cultures and Climates is to provide information that supports and promotes diversity, inclusivity, and equity in the international plant sciences community so that it grows to more accurately reflect that of our larger, global society.
At CCC, on Plantae supported by ASPB, you will find impactful literature about culture and gender matters; information about programs and initiatives that focus on increasing and sustaining diversity in plant science; and a safe space to learn more about one another and to discuss topics around diversity, equity, and inclusion that impact our community.
NAASC collaborates on this initiative though invovement of several members as well as the NAASC section of the webpage:
https://plantae.org/education/changing-cultures-and-climates/#naasc-initiatives

NAASC #DiversifyPlantPubs is simple and straight-forward. The NAASC DiversifyPlantSci Twitter account [@DiversifyPlants] (https://twitter.com/diversifyplants) re-tweets scientific articles, including pre-prints, written by scientists who self-identify as plant science researchers with diverse identity(ies).
-
- If you are Black, Indigenous, Latin@x, any race or ethnicity where you identify as a person of color, a woman, LGBTQIA+, occupy a social space or have an identity that is under-represented, minoritized, or less/in-visible in plant sciences--and you are publishing in the plant sciences--just @ us.
- Include the link to the paper and any pics or biographical information. We will quickly scan the paper to make sure the submitter is an author and that the paper is relevant to plant sciences, and then we will re-tweet.
- Please note that the author that self-describes as bringing diversity should submit (not their co-authors)
- You can also submit papers to DiversifyPlantPubs by direct message to us @DiversifyPlantSci, or by emailing This email address is being protected from spambots. You need JavaScript enabled to view it.
-
We want to promote, lift up, and disseminate the research of plant scientists whose identities diversify our community. We don't want to wait for the plant science community to increase its diversity and representation to make these contributions more visible.
This DiversifyPlantSci DiversifyPlantPubs initiative is inspired by, and modeled after, the EEB Papers of Color initiative from our colleagues in Ecology, Evolution, & Behavior (EEB); check them out @ EEB_POC and https://github.com/jhpantel/EEB_POC/blob/master/EEB_POC.pdf
The events of the past week provide another searing reminder that we have a great deal of work to do before we realize a world where everyone has a full, healthy life and the opportunity to fulfill their potential.
The deaths of George Floyd, Breonna Taylor, Tony McDade, Ahmaud Arbery, and David McAtee are only the most recent and most violent examples of what Black Americans experience every day. While it might seem to some that academia and the sciences provide a space of refuge, racism is prevalent within the academy, and it is hurting valued members of our community. We acknowledge that for many of us, including our mentees, it may not be possible–or even appropriate–to focus on science right now.
As a group elected to represent the community of Arabidopsis researchers in North America, we:
-
- Express our anger, sadness, and disgust at current events, including the violent response to peaceful protesters by police and the inflammatory language of many in leadership roles. NAASC strongly rejects racism and discrimination in any form.
- Reach out to our Black colleagues, trainees, and students to express support and solidarity and empathy. We stand with the Black community, and extend our love and concern to other communities that experience injustice.
- Will continue our efforts to raise up and center minoritized voices. This includes using DiversifyPlantSci (https://tinyurl.com/DiversifyPlantSci) as one of several resources for identifying potential speakers for seminars, meetings and candidates for leadership roles, and sponsoring focused activities to support, mentor, and promote the advancement of under-represented minorities.
- Reiterate that anti-racism work is explicitly part of NAASC’s mission, and should be for all scientists. We reaffirm our commitment to doing the work needed to break down structural inequities. In this article, we provide some resources for those with privilege. We commit ourselves to providing more resources in the future.
We call on our colleagues who are not themselves in marginalized communities to reflect on what specific actions you can take to support those in the Black community. In addition, we ask our entire NAASC constituency to provide feedback for how we might better serve and support you.
NAASC Committee for Diversity and Equity
This email address is being protected from spambots. You need JavaScript enabled to view it.
Click to see the ICAR 2020 Code of Conduct Summary
ICAR 2020 Code of Conduct (applied to ICAR 2021-Virtual)
NAASC developed this code of conduct in 2019 for the 31st International Conference on Arabidopsis Research (ICAR) scheduled for July, 2020. ICAR 2020 was then postponed due to global Covid-19 pandemic, and converted to ICAR 2021-Virtual.
This code of conduct includes on-site processes that became obsolete once the conference became a virtual meeting.
However, as we have developed this document also as a resource for others that may wish to develop their own conference code of conduct, we share this information freely in its original form and invite people to use and/or adapt the document as you wish.
We ask that you abide by the ICAR 2020 Code of Conduct References, Re-use and Acknowledgement Policy that is included preceding the citation list.
Summary of ICAR Code Expectations and Consequences of Violations
(1) Expected Behavior
- All participants in our events and communications are expected to show respect and courtesy to others.
- All interactions should be professional regardless of platform: either online (including via social media) or in-person.
- Be respectful of different viewpoints and experiences.
- Use welcoming and inclusive behavior.
- Show courtesy and respect towards other participants, including but not limited to: conference attendees, speakers, staff and all University personnel, including staff, students, etc.
(2) Examples of inappropriate conduct and behavior prohibited by the ICAR Code
Identity-based discrimination: written or verbal comments which have the effect of excluding people on the basis of membership of any specific group such as race, gender, sexual orientation, gender presentation, etc., as described in the Code.
- Causing someone to fear for their safety, such as through stalking, following, or intimidation
- Violent threats or language directed against another person.
- Sexual harassment or misconduct: unwelcome sexual attention; nonconsensual or unwelcome physical contact, offensive or degrading remarks, sexist slurs, or demeaning comments.
- The display of sexual or violent images.
- Incivility: Sustained disruption of talks, events or communication; insults or put downs; sexist, racist, homophobic, transphobic, ableist, or exclusionary jokes; excessive swearing.
- Continuing to initiate interaction (including photography or recording) after being asked to stop.
- Publication of private communication (text, verbal, images) without consent.
(3) Consequences of Unacceptable Behavior/ Violations of ICAR Code
Following review as described in the ICAR Code, the consequences of violations will include one or more of the following actions:
- Warned/asked to stop
- Removed from meeting without warning or refund
- Prohibited from attending future ICARs
- Employer notified
- Reported to law enforcement
- Retaliation for reporting harassment is also a violation of this policy. This provision extends to bystanders.
NAASC Members of the North American Arabidopsis research community are nominated and elected by the community to serve on the NAASC since 1992.
The history, structure (evolving) and membership of NAASC are described below:
During the first years of the Arabidopsis Genome Project, an ad hoc committee was formed to forge relationships and foster communication among the groups and countries world-wide who were involved in the genome sequencing effort. It was determined that the committee should be made up of three representatives from North America, two representatives each from continental Europe and the United Kingdom, and one representative each from Australia and Japan. These representatives would be elected by the groups that they would represent, and they would serve terms of three years. This committee was known as the Multinational Science Steering Committee. In February of 1992, in response to the need for elected North American representatives to the Multinational Science Steering Committee, Howard Goodman, Elliot Meyerowitz and Chris Somerville called for the formation of a North American Arabidopsis Steering Committee (NAASC). In the first election, six North American Arabidopsis researchers were elected.
In its first year, the NAASC dealt with a number of issues including the decision to have a National Arabidopsis meeting in Ohio in 1993, determining who should represent North America on the Multinational Science Steering Committee, and advising the NSF and other funding agencies of the community's needs for database services.
It was additionally determined that the committee would consist of six members and that members would serve for three years. Two new members would be elected annually via the Arabidopsis Newsgroup , and two members would retire.
Since that time, the NAASC has evolved into the main organizing and fundraising body for the International Conference on Arabidopsis Research when it is held in North America. The conference is now held annually and rotates between North America, Europe, and Austral-Asia.
The NAASC also collaborates with MASC members that volunteer to host the annual ICAR. NAASC solicits funds to help North American junior scientists travel to these international meetings. Since 2004, the NAASC has applied for funding to allow underrepresented minorities, and scientists from Historically Black Universities and Minority-Serving Institutions in the United States to fully participate in the Annual Arabidopsis meetings. Additionally, the NAASC serves as a liaison between members of the community and government and not-for-profit granting agencies and provides representation of the community to service facilities.
In 2000, the members of the NAASC unanimously voted to amend the process of election and alter the term of service for members to coincide with the annual conference rather than the calendar year. It was felt that this alteration would help to ensure continuity in the committee. In 2003, the process was again amended, in the following ways:
Each member of the committee will serve for four full years, to further aid in continuity in the committee (August 29, 2017 Update to NAASC Term of Service: To provide better continuity and shared knowledge for NAASC-organized ICARs the term of service increased from four to five years. All NAASC elections beginning with fall 2017 will designate 5 year terms for new NAASC members.)
Members of the North American Arabidopsis research community who have served previously on NAASC may be re-nominated for the election, and if elected, may serve another term on the committee.
NAASC Structure

NAASC COMMITTEES: DESCRIPTIONS, MEMBERSHIP
NAASC
Description: The North American Arabidopsis Steering Committee (NAASC), an elected body, was founded in 1992 to provide North American representation to the Multinational Arabidopsis Steering Committee (MASC) and to forge relationships and foster communication among the groups and countries world-wide that are involved in research, education, and outreach using Arabidopsis. NAASC organizes and raises funds for North American International Conferences on Arabidopsis Research (ICAR), and supports North American researchers to participate in additional ICARs. NAASC provides leadership to develop, secure funding for, and implement community-serving projects and activities and enable opportunities to support the full diversity of the Arabidopsis community, particularly in North America. http://arabidopsisresearch.org/index.php/en/naasc
Membership: NAASC has 10 elected members with two new members elected annually by the North American Arabidopsis community; members serve a 5-year term. Eligibility to serve on NAASC: Persons at the postdoctoral scholar level and higher, residing and working on Arabidopsis-related research and/or education and/or training at a North American University or Institution.
DEILC
Description: The NAASC Diversity, Equity and Inclusion Leadership Committee was founded in 2020 to more effectively and inclusively coordinate efforts between NAASC and its subcommittees, and to expand community input and participation in areas of diversity, equity and inclusion of multiple identities of the Arabidopsis community. DEILC will ensure NAASC uses a lens of social and racial equity and inclusion during activity development and implementation; particular attention is paid to ensure that historically less-resourced racial and ethnic groups, and early career scholars, have representation in NAASC activities.
ISS
Description: The NAASC Inclusivity Scholars Subcommittee (ISS) was founded in 2013 (as the Minority Affairs Committee, MAC) to increase the presence of historically under-represented ethnic and racial groups in the fields of Arabidopsis research, education, and outreach, as well as to collaboratively address issues they face as plant scientists and in society. ISS seeks to coordinate activities related to promoting diversity in plant sciences, primarily through cultivating programs and communities around the ICAR Inclusivity Scholars Program (ISP, formerly the Under Represented Minority (URM) Travel Award Program). ISS will continue to provide resources, opportunities, mentoring and training to current and past members of this ISS cohort.
Membership: ISS is co-chaired by two members of the Arabidopsis community, currently this is Cris Argueso (Colorado State University), and the community member slot is vacant. The past co-chairs are Siobhan Brady (UC Davis) and Terri Long (NCSU), who served for ~10 years to support ISS. Their primary focus is coordinating and enabling the ISP and associated cohort, and NAASC is truly grateful for their service and dedication to the Scholars. Past ICAR awardees (previously “URM cohort members”) are eligible to join ISS; additional interested North American Arabidopsis scientists or educators whose experiences and background are relevant can also apply to join ISS. ISS welcomes allies to participate in ISS activities, and especially promotes action by allies to challenge power structures in their own institutions and groups that seek to oppress or disenfranchise historically under-represented ethnic and racial groups. Members will be asked to provide brief biosketches that focus on their interests in diversity, equity and inclusion (as well as research and educational interests).
To Apply: Email* these materials: (1) Statement on interests: up to 1 page describing your scientific, educational, and/or outreach interests that are relevant to Arabidopsis and/or other plant sciences and why you wish to serve on the ISS including your interest, activities, and/or priorities for increasing the presence of under-represented ethnic and racial groups in plant sciences. Please indicate whether you are yourself Black, African American, LatinX, or Indigenous; or alternatively, if you consider yourself an ally from a non-minority/represented group. (2) Brief (2-3 pages) CV or resume to* This email address is being protected from spambots. You need JavaScript enabled to view it., Subject line: Application for ISS. For more information, email questions to preceding email address.
ECSS
Description: The NAASC Early Career Scholars Subcommittee (ECSS) was founded in 2020 to 1) ensure that Early Career Scholars (ECS) have representation in NAASC activities at all stages of activity development and implementation, and 2) to provide a forum where we can collaboratively address issues specific to ECS. ECSS liaises with NAASC via DEILC.
Leadership: ECSS is co-chaired by Dior Kelley (NAASC, Iowa State Univ.) & Glen Uhrig (community member, Univ. of Alberta). Additional community members are Sixue Chen (Univ. of Mississippi) and Ling Li (Mississipi State Univ.)
Membership: ECSS will be co-chaired by two North American Arabidopsis community members, one of whom should be an elected member of NAASC, and that themselves are ECS, defined as: undergraduate or graduate students, postdoctoral scholars, junior faculty (pre-tenure), or others (e.g., research scientists and faculty in non-tenure track positions). All ECS are defined as within 10 years of securing a doctorate in a relevant field or 15 years of securing a master’s degree (if that is their terminal degree). Co-chair terms: (1) elected NAASC member: equal to their term on NAASC unless they are replaced prior to end of their term. (2) If not an elected NAASC member: two years, eligible for renewal upon NAASC approval. Eligible to join ECSS: past North American-based NAASC early career travel awardees and any interested North American Arabidopsis scientists or educators whose experiences and background are relevant to ECSS. Applicants/Members will be asked to provide brief biosketches that focus on their interests in diversity, equity and inclusion (as well as research and educational interests).
To apply: Email these materials: (1) Statement on interests: up to 1 page describing your scientific, educational, and/or outreach interests that are relevant to Arabidopsis or other plant sciences and why you wish to serve on the ECSS. (2) Brief (2-3 pages) CV or resume listing your educational and professional achievements to This email address is being protected from spambots. You need JavaScript enabled to view it., Subject line: Application for ECSS. For more information, email questions to preceding email address.
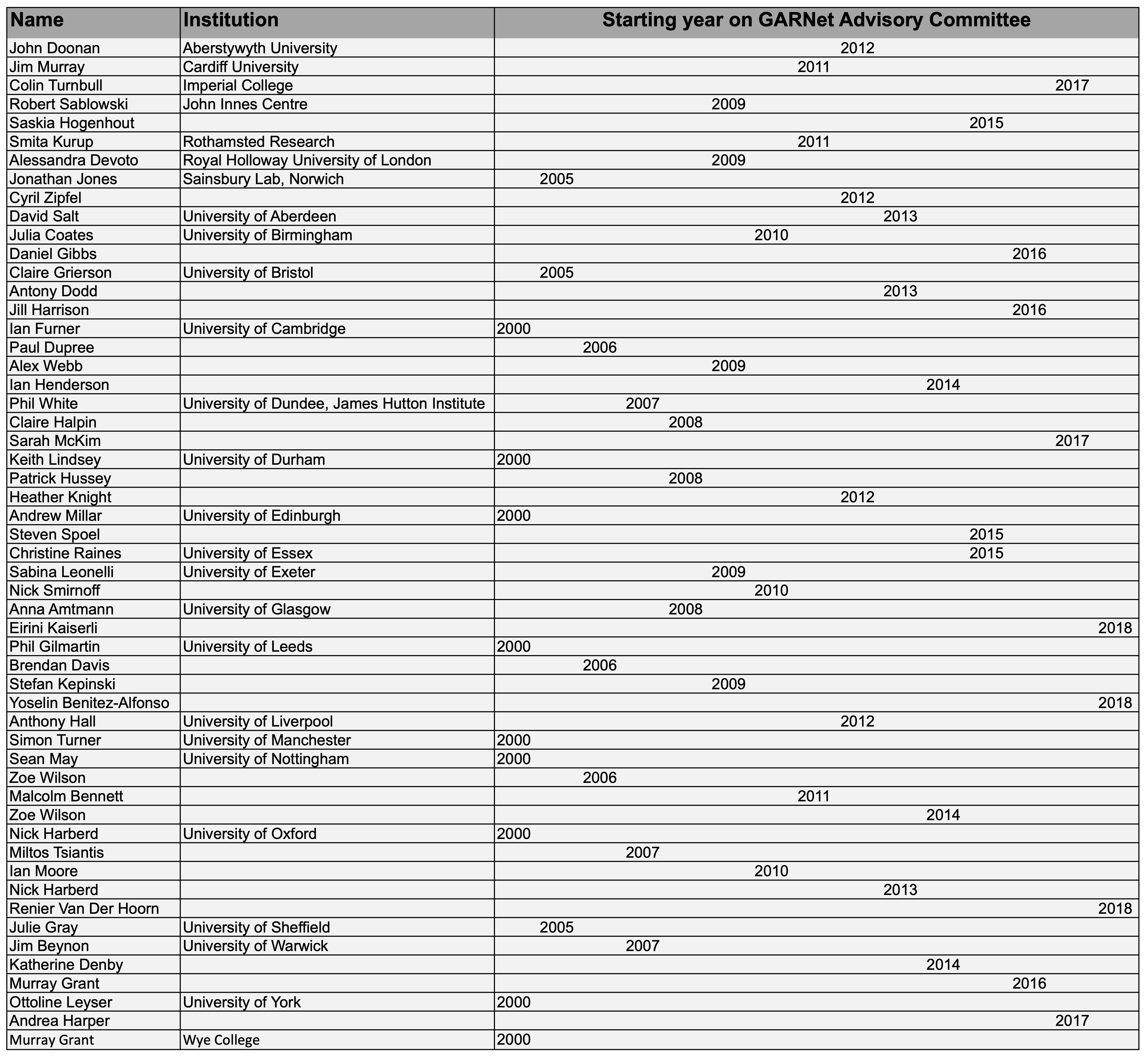
Members of the North American Arabidopsis research community have been extremely generous in their willingness to serve on the NAASC. Since the initial call for an elected committee in 1992, 60+ researchers/educators have served on the committee.
NAASC members, past and present, are listed below:
- Elliot Meyerowitz 1992 - 1994
- Chris Somerville 1992 - 1994
- Fred Ausubel 1992 - 1995
- Joe Ecker 1992 - 1995
- Joanne Chory 1992 - 1996
- David Meinke 1992 - 1996
- Gloria Coruzzi 1994 - 1997
- Mark Estelle 1994 - 1997
- Pam Green 1995 - 1998
- Rob Last 1995 - 1998
- Rick Amasino 1996 - 1999
- Daphne Preuss 1996 - 1999
- Jeff Dangl 1997 - 2000
- Detlef Weigel 1997 - 2000
- Chuck Gasser 1998 - 2001
- Steve Kay 1998 - 2001
- Kathy Barton 1999 - 2002
- Mary Lou Guerinot 1999 - 2002
- Peter McCourt 2000 - 2003
- Mike Sussman 2000 - 2003
- Bonnie Bartel 2001 - 2005
- Eric Richards 2001 - 2005
- Greg Copenhaver 2002 - 2006
- Brenda Winkel 2002 - 2006
- Philip Benfey 2003 - 2007
- Rob McClung 2003 - 2007
- Judith Bender 2004 - 2008
- Xing-Wang Deng 2004 - 2008
- Joe Kieber 2005 - 2009
- Xuemei Chen 2005 - 2009
- Caren Chang 2006 - 2010
- Julian Schroeder 2006 - 2010
- Scott Poethig 2007 - 2011
- George Haughn 2007 - 2011
- Mark Estelle 2008 - 2012
- Jane Glazebrook 2008 - 2012
- Xinnian Dong 2009 - 2013
- Blake Meyers 2009 - 2013
- Dominique Bergmann 2010 - 2014
- Wolf Frommer 2010 - 2014
- Nicholas Provart 2011 - 2015
- Jose Alonso 2011 - 2015
- Siobhan Brady 2012 - 2016
- Keiko Torii 2012 - 2016
- Sally Assmann 2013 - 2018 (extended for new 5 year term)
- Erich Grotewold 2013 - 2018 (extended for new 5 year term)
- Doris Wagner 2014 - 2018
- Richard Vierstra 2014 - 2018
- Jose Dinneny 2015 - 2020
- Elizabeth Haswell 2015 - 2020
- Roger Innes 2016 - 2021
- Peter McCourt 2016 - 2021
- Sean Cutler 2017 - 2021
- Jennifer Nemhauser 2017 - 2022
- Federica Brandizzi 2018 - 2023
- Anna Stepanova 2018 - 2023
- Siobhan Braybrook 2019 - 2024
- Keith Slotkin 2019 - 2024
- Cris Argueso 2020 - 2025
- Adrienne Roeder 2020 - 2025
- Dior Kelley 2021 - 2026
- Glora Muday 2021 - 2026
- Mentewab Ayelew 2022 - 2027
- Liang Song 2022 - 2027
Last modified on November 4 2022